Diving into the fascinating world of atomic structures, it’s impossible to overlook the significance of ca electron configurations. They’re the key to understanding 
Calcium, with atomic number 20, has an intriguing ca electron configuration that plays a vital role in its chemical properties. It’s this configuration that makes Calcium a key player in various industrial applications and biological processes.
Ca Electron Configuration
Circling back to the core concept that underpins our discussions on elemental behaviors — ca electron configuration. This forms the bedrock for understanding Calcium’s reactivity, stability, and utility in various applications.
What is Ca Electron Configuration?
Briefly, ca electron configuration refers to the specific distribution or arrangement of electrons in atom’s electron shell which effectively dictates its chemical behavior. It’s based on the principal of the Pauli Exclusion Principle, where no two electrons in an atom can share the exact same quantum state. This fact fundamentally underpins the arrangement of electrons around an atom.
Importance of Ca Electron Configuration
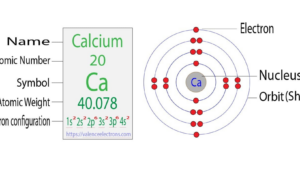
For instance, Calcium’s reactivity with other elements is often a direct result of its ca electron configuration. It readily loses its two outermost electrons (from the 4s orbitals) to achieve a more stable ca electron configuration, typically behaving as a +2 ion in chemical reactions. This pattern of behavior is critical to understanding Calcium’s noteworthy role in biological functions and industrial applications, from the creation of strong bones and teeth to the manufacture of cement.
Sublevels and Orbitals
Understanding ca electron configurations isn’t just about knowing an element’s atomic number or its position on the periodic table. It’s also springboarding into the intricacies of atomic structures typically represented by sublevels and orbitals.
Sublevels in Ca Electron Configuration
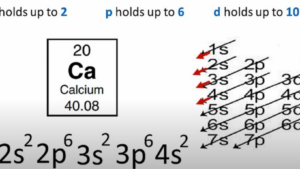
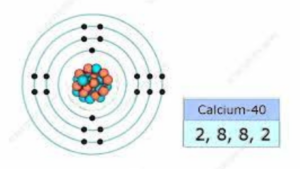
For instance, Calcium (Ca), with an atomic number of 20, has four sublevels. At the lowest energy level, – the 1s sublevel, there are two electrons. As for the second energy level, it hosts both the 2s sublevel (holding 2 electrons) and the 2p sublevel (holding 6 electrons). The third energy level mirrors the second, with two electrons in the 3s sublevel and six in the 3p sublevel. Finally, the fourth level merely contains the 4s sublevel, which holds two electrons.
Orbitals in Ca Electron Configuration
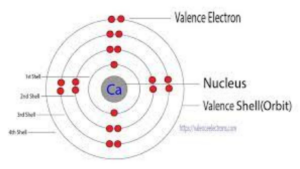
Each sublevel is made up of a certain number of orbitals. An s sublevel has one orbital – can accommodate two electrons. A p sublevel has three orbitals – can house six electrons. A d sublevel contains five orbitals – accepts 10 electrons. Lastly, an f sublevel with seven orbitals can snug 14 electrons.
Like previously mentioned, Calcium’s electron configuration includes the s and p orbitals up to its fourth energy level. This configuration dictates how Calcium behaves in chemical reactions, often losing its two outermost electrons to achieve stability.

Point to remember:
- Sublevels and orbitals serve as a snapshot of an atom’s internal landscape.
- Calcium’s electron configuration directly impacts its chemical behavior.
That’s it for the subsections “Sublevels in Electron Configurations” and “Orbitals in Electron Configurations”. Stay tuned, as we continue to delve deeper into the fascinating domain of electron configurations.
Writing Ca Electron Configuration
Aufbau Principle
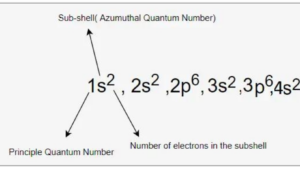
Hund’s Rule
Pauli Exclusion Principle
Finally, the “Pauli Exclusion Principle” rounds off this triad of important principles 
Understanding these principles aids in decoding the language of ca electron configurations, providing insights into the unique behavior of electrons within atoms. As we continue to delve into the specifics of calcium’s electron configuration in subsequent sections, keep in mind the regulations imposed by these guiding principles.
What You Need To Know
It’s clear that ca electron configurations aren’t always predictable. Transition Metals like Chromium and Copper, along with Lanthanides and Actinides, often defy the expected patterns. These exceptions underscore the complexity of ca electron configurations and the challenges they present to our understanding of atomic structure and reactivity. They also highlight the limitations of the Aufbau Principle, Hund’s Rule, and the Pauli Exclusion Principle.

